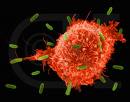
London: Scientists at London’s Imperial Colleage have captured the body’s immune cells at work.
The mechanism used by ‘Natural Killer’ immune cells in the human body to distinguish between diseased cells, which they are meant to destroy, and normal cells, which they are meant to leave alone, is revealed in new detail in research published in PLoS Biology.
Understanding how this aspect of the body’s natural defences works could help medical researchers develop new ways of boosting these defences to treat disease.
Natural Killer (NK) cells – a type of white blood cell – are a major component of the human body’s innate immune system. Over 1,000 NK cells are found in every drop of blood. They provide a fast frontline defence against tumours, viruses and bacterial infections, by latching onto and killing cells in the human body that are cancerous or are infected with a virus or a bacterial pathogen.
On their journey round the human body NK cells regularly latch onto normal non-diseased cells too, before moving off, leaving them unharmed. Previously, the process by which NK cells made the right decision to kill or not kill another cell was unclear.
Now, the team of researchers from Imperial College London have used high speed microscopy imaging techniques to observe the NK cell decision making process in action. This has revealed striking differences in the behaviour of NK cells when interacting with healthy or diseased cells.
The outcome of the decision making process is determined by how receptors on the surface of the NK cell interact with proteins on the surface of the captured cell. Every NK cell has two types of surface receptors – activators, which turn the killing mechanism ‘on’ and inhibitors which turn the killing mechanism ‘off’.
Professor Davis and his colleagues discovered that if a captured cell is diseased or cancerous, it interacts with a large number of the NK cell’s activating receptors, which makes the NK cell stop dead in its tracks and spread out over the captured cell. During this spreading process the NK cell continuously reads the ‘on’ and ‘off’ signals from its surface contact with the captured cell. If the ‘on’ signals dominate, the NK cell prolongs contact with the captured cell and eventually kills it.
Conversely if the captured cell is healthy, it interacts with more of the NK cell’s inhibiting receptors – and fewer of its activating receptors – meaning that the ‘off’ signals dominate and the ‘stopping and spreading’ process does not occur, allowing the NK cell to quickly move off in search of a new target.
Principal investigator of the new study, Professor Dan Davis from Imperial College London’s Department of Life Sciences, explains:
“Scientists have known for a long time that the proteins on the surface on Natural Killer cells are involved in answering the ‘to kill or not to kill?’ question, but we’ve not known exactly how these molecular cues are translated into the correct response. Our research has shown that information gleaned from its surface receptors tells the Natural Killer cell whether to stop patrolling and commence killing, or to move off quickly, and harmlessly, in search of another target.”
Dr Fiona Culley, lead author of the study from the National Heart and Lung Institute at Imperial, says that finding out how NK cells use this process to sift out diseased cells from normal ones paints a very clear picture of how these cells do their vital work:
“Considering that NK cells play such an important part in our immune response to cancer and disease, relatively little is known about their functionality – how exactly they work and how they interact with the cells they encounter inside us. This study adds significantly to our understanding of how Natural Killer cells distinguish between healthy and diseased cells.”
The research was funded primarily by the Lister Institute for Preventative Medicine and the Medical Research Council, with additional support from the Biotechnology and Biological Sciences Research Council, the Royal Society and the Wellcome Trust.
